2189
Combining Arterial Blood Contrast with BOLD improves fMRI laminar specificity.1Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 2MR-Methods group, MBIC, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 3Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands
Synopsis
BOLD fMRI is widely applied in human neuroscience, but is limited in its spatial specificity compared to cerebral blood volume approaches due to its venous bias. Here we added cerebral blood-volume weighting based on magnetization transfer (Arterial Blood Contrast) to a BOLD submillimeter acquisition at 7T. Adding Arterial Blood Contrast helped differentiate the deep and superficial cortical responses in the human primary motor cortex. The results suggest that combining Arterial Blood Contrast with BOLD can improve the fMRI spatial specificity while retaining high sensitivity.
Introduction
fMRI allows imaging the neural underpinnings of behavior in-vivo and has seen therefore widespread application1. fMRI contrast is typically produced using blood deoxyhemoglobin as an endogenous contrast agent (BOLD), which biases spatial sensitivity towards vein-rich areas, like the superficial layer of the cortex2. Cerebral blood volume (CBV) approaches are more spatially specific, showing increased intracortical functional contrast3, but are less sensitive. Arterial Blood Contrast (ABC) is a newly-introduced technique4 that produces a CBV-weighted functional signal by preferentially suppressing the tissue through the saturation of the macromolecular pool and the transfer of spin coherence to the free water pool5. The spatial-specificity benefits of ABC are currently unexplored. In this study, we employ a task paradigm thought to elicit intracortical functional contrast6 to examine the specificity of a submillimeter BOLD technique with added ABC-weighting.Methods
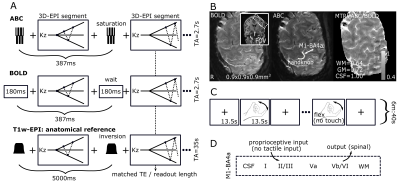
All six participants (four female) were scanned in a Philips Achieva 7T with a 8Tx/32Rx whole-head coil (Nova Medical). The participants performed two counterbalanced runs of a finger-flexing task (right index finger, ON=13.5s, OFF=13.5s, run-duration=6min:40s), while a segmented submillimeter 3D-EPI was recorded (FOV=120x131x31mm3, voxel-size=0.9mm isotropic, TA/TR/TE=2700ms/52ms/18ms, flip-angle=20o, SENSEy/z=3.5/1.5, partial-Fouriery/z=0.7, shot-duration=218ms) (Fig.1A,B). Between each segment either a saturation train (7 hard rectangular on-resonance phase-modulated pulses, B1=10μT, total duration=6ms, 95% SAR limit4,7) or an equivalent wait was inserted, to achieve ABC- or BOLD-weighting. The ratio between ABC and BOLD showed 64% remaining signal in white-matter (WM) and 72% in grey-matter (GM). A distortion-matched, T1-weighted 3D-EPI was obtained by replacing the saturation train with an adiabatic inversion pulse (TI=1100ms). The 3D-EPIs were placed perpendicular to the left primary motor cortex to optimally visualize the handknob (Figure 1B), including the lateral side (M1-BA4a) that has been reported to show a double-stripe CBV fMRI response to finger flexing (Fig.1C,D)6.The fMRI data were motion-corrected and projected to the T1-weighted-EPI with a single interpolation. A GLM (finger-tapping>rest) was fitted (FSL6.0.1) and the percent signal change was calculated. Cortical depth profiles of the GM from WM to pial surface were extracted from a manually drawn ROI in M1-BA4a (LAYNII8). For two participants, a second session was recorded on a different day to examine reproducibility.Results
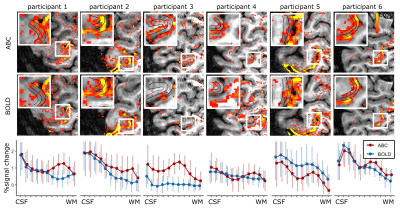
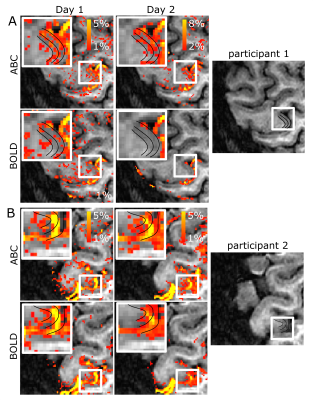
Cortical depth sampling of the M1-BA4a during finger flexing showed an increased percent signal change close to WM for ABC compared to BOLD, resulting in a consistent between participants activation bump in deep GM (peak percent signal change ranging from 0.75 to 1.3% between participants in deep GM). This stripe pattern was visually obvious in the unsmoothed data in 4 out of 6 participants (Fig.2A-C). In the superficial GM, a second peak was not readily visible, though in 2 participants a second peak was observed in both BOLD and ABC.The ABC response, including the stripe pattern, was reproducible in different days (Figure 3A). ABC and BOLD average timecourses were similar, with a potentially slightly earlier peak in the outer and middle GM for ABC compared to BOLD within M1-BA4a (Fig.4A-C).
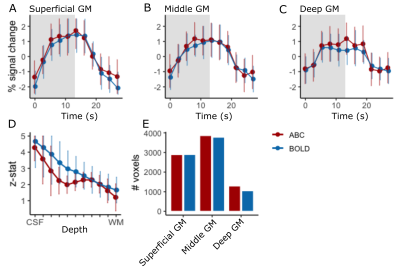
The mean cortical depth response of ABC across participants within M1-BA4a showed similar sensitivity to BOLD with a small z-stat reduction in mid-GM reflecting the observed stripe pattern (Fig.4D). Note that the reproduction of this stripe pattern in ABC in both percent signal change and z-stats suggests that this not driven by a reduced baseline signal but rather a task-locked change. The mean number of active voxels (p<0.05) across the whole slab was similar between BOLD and ABC, implying similar sensitivity, with a slight increase in deep GM (Fig.4E).
Discussion
Increasing the fMRI spatial specificity in the human cerebral cortex is an important target for human neuroscience. Here, we showed that adding ABC-weighting elicited a differential deep and superficial cortical response that was not detected with standard BOLD weighting in most participants. The ABC approach offers high sensitivity, straightforward implementation and high temporal efficiency, despite the SAR-constrained 7T environment. High-resolution ABC may therefore have widespread application in in-vivo neuroscience.The ABC-weighting as implemented here is added to the underlying BOLD contrast. Preferentially saturating the GM tissue may increase spatial specificity and sensitivity due to an increased arteriole and capillary contribution through the suppression of the (negative) tissue signal change, as well as due to increased water signal. This is supported by the potential slightly earlier peak of the ABC response in the superficial and middle GM, in agreement with added arterial weighting, though experiments with longer interstimulus intervals would be needed to test this. The perfusion of saturated water from the tissue to the capillaries and further downstream may further decrease the venous contribution, as well as a partial direct saturation of the (shorter T2) venous component.
Despite the increased specificity, a substantial venous bias remains in the current implementation, as evidenced by the close agreement between BOLD and ABC close to the pial surface. Exploring the TE space to reduce the venous contribution (either through fully center-out readouts or at TEs where the short T2* venous signal has decayed) may further increase specificity in the future.
Conclusion
BOLD fMRI is widely applied in human neuroscience, but is limited in its spatial specificity. Here, we showed that adding ABC-weighting to a submillimeter BOLD-weighted 7T acquisition increases intracortical contrast, while retaining high sensitivity.Acknowledgements
This study was supported by an NWO TTW VIDI grant (VI.Vidi.198.016)References
1. Glover, G.H., Overview of functional magnetic resonance imaging. Neurosurg Clin N Am, 2011. 22(2): p. 133-9, vii.
2. Uludag, K., B. Muller-Bierl, and K. Ugurbil, An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage, 2009. 48(1): p. 150-65.
3. Huber, L., et al., Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications. Neuroimage, 2018. 164: p. 131-143.
4. Schulz, J., et al., Arterial blood contrast (ABC) enabled by magnetization transfer (MT): a novel MRI technique for enhancing the measurement of brain activation changes. 2020: Biorxiv.
5. Kim, T., K. Hendrich, and S.G. Kim, Functional MRI with magnetization transfer effects: determination of BOLD and arterial blood volume changes. Magn Reson Med, 2008. 60(6): p. 1518-23.
6. Huber, L., et al., High-Resolution CBV-fMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1. Neuron, 2017. 96(6): p. 1253-1263 e7.
7. Priovoulos, N., et al., Submillimeter Arterial Blood Contrast fMRI at 7T, in Proc. Intl. Soc. Mag. Reson. Med. 29. 2021: Paris.
8. Huber, L.R., et al., LayNii: A software suite for layer-fMRI. Neuroimage, 2021. 237: p. 118091.
Figures